Cancer Initiative and Genome Integrity
How does it start?
How does it progress?

How do we predict?

How do we treat?
GICI: Genome Integrity and Cancer Initiative
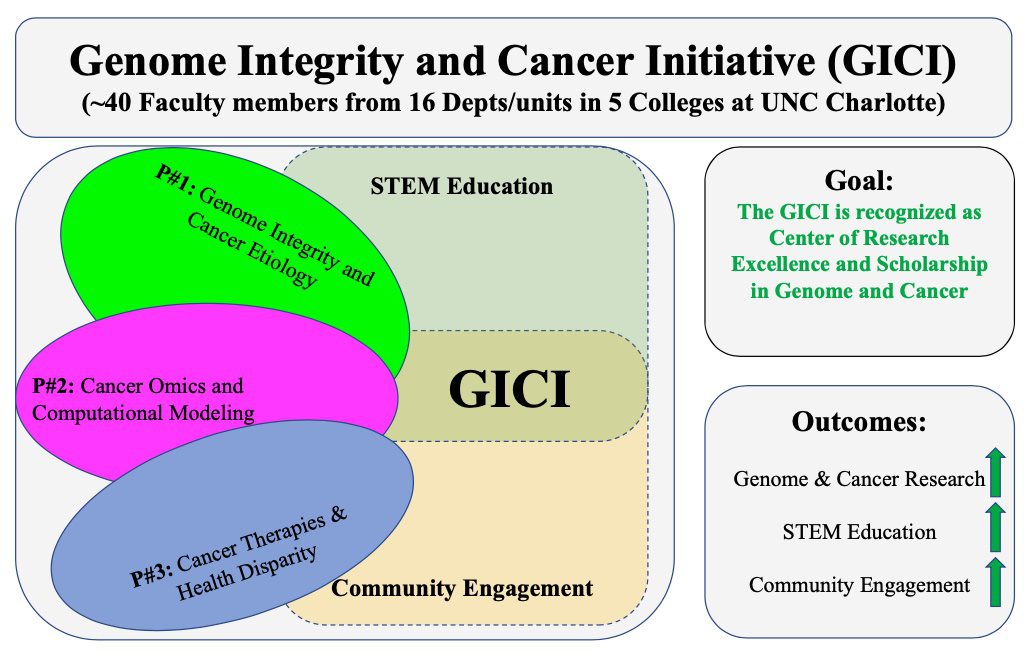
The Genome Integrity and Cancer Initiative (GICI) is composed of three programs: (1) Genome integrity and cancer etiology (Lead- Shan Yan); (2) Cancer-omics and computational modeling (Co- Lead: Jun-tao Guo); (3) Cancer therapeutics and health disparity (Co-Lead: Didier Dréau) as well as STEM education and community engagement and outreach. The GICI will foster genome, omics, and cancer research, to support translational efforts to improve cancer treatment and community health, and promote STEM education and community engagement and outreach activities.
The GICI aims (1) to pursue excellence in cancer scholarship and research that address key fundamental cancer genetic, cellular and therapeutic challenges; (2) to pursue excellence in education by leveraging synergies between research and learning across our university community of scholars to offer undergraduate and graduate students both research experiences and innovative teaching; and (3) to promote community engagement and outreach activities in the local greater Charlotte region. Overall, GICI is fully committed to research excellence and scholarship as well as broad participation in both existing and emerging excellence. GICI is poised to make further strides in cancer research by harnessing the promises of (1) unity of the connecting participants (40 Faculty from 16 Depts/Units in 5 Colleges); (2) targeted individual and cancer (e.g., exercise, diet, genetic) factors; (3) cancer literacy and community awareness through outreach; and (4) strengthen the relationships with other regional, state, and national institutions. GICI will build on cutting-edge cancer research and embrace new pedagogy that fosters innovation in cancer research and education toward a recognized top-tier genome and cancer research center of excellence, through (1) the development and nurturing of a diverse intellectual research team that promotes cancer research investigation using molecular, cell, organism, model and community approaches; (2) the support of multi- and inter-disciplinary research opportunities/applications including those addressing cancer health disparities; and (3) the active participation in transforming STEM education and student involvement in cancer research and addressing cancer challenges at the local and national levels.
Upcoming Events
Dr. Shan Yan established the Exchange Group Charlotte Biology and Biotechnology with the North Carolina Biotechnology Center to bring the Distinguished Speaker in Genome Integrity Seminar series to campus. Upcoming dates:
- OCTOBER 8, 2021. Jianjun Zhao, PhD, Department of Cancer Biology, Lerner Research Institute, Cleveland Clinic. Cisplatin Upregulates Apurinic/Apyrimidinic Endonuclease 2 (APE2) Binding to Myosin Heavy-Chain 9 (MYH9), Provoking Mitochondrial Fragmentation and Acute Kidney Injury.
- NOVEMBER 5, 2021. Marcus Smolka, PhD, Department of Molecular Biology and Genetics, Cornell University. Boosting and Braking DNA Repair in Cancer.
Genome Integrity and Etiology Group (GIG)
GIG is a collaborative group of investigators involved in interdisciplinary research to understand DNA replication, recombination, damage and repair mechanisms in cancer and other human diseases. The GIG at UNC Charlotte aims to develop novel technologies and methodologies to investigate genome integrity. In addition to publishing high impact research, the GIG aims to provide an enriching training environment for Postdoctoral researchers, Graduate and Undergraduate students. Core members include leader Dr. Shan Yan (Molecular mechanisms of genome integrity and cancer etiology), Dr. Christine Richardson (DNA recombination and repair and cancer biology), Dr. Andrew Truman (Role of Molecular chaperones in the DNA damage response), Dr. Kausik Chakrabarti (RNA regulation in human pathogens and telomerase), and Dr. Junya Tomida (DNA repair, kinase regulation, cancer carcinogenesis and metastasis). This group meets every month for presentations by lab members, discussions and collaborations. Main Areas of Research in Program #1:
- Genome integrity, DNA repair, and DNA damage response
- DNA/RNA damage and cancer etiology
- Cellular signaling and molecular mechanisms
- Organismal, ecological and environmental analysis of genome integrity
Chakrabarti Lab
For more about Dr. Chakrabarti’s lab, visit his research lab webpage.
Research projects
- Structure and function of RNA and ribonucleoprotein complexes.
- Telomerase regulation and genome integrity in unicellular pathogens.
- Post-transcriptional mRNA processing in cell proliferation and cancer.
Representative Publications
- Dey A, Chakrabarti K. Current Perspectives of Telomerase Structure and Function in Eukaryotes with Emerging Views on Telomerase in Human Parasites. Int J Mol Sci. 2018;19(2). Epub 2018/01/25. doi: 10.3390/ijms19020333. PMID: 29364142;
- Ares M, Jr., Chakrabarti K*. Stuttering against marginotomy. Nat Struct Mol Biol. 2008;15(1):18-9. Epub 2008/01/08. doi: 10.1038/nsmb0108-18. PMID: 18176550.
- Sandhu R, Sanford S, Basu S, Park M, Pandya UM, Li B, Chakrabarti K*. A trans-spliced telomerase RNA dictates telomere synthesis in Trypanosoma brucei. Cell Res. 2013;23(4):537-51. Epub 2013/03/13. doi: 10.1038/cr.2013.35. PubMed PMID: 23478302; PMCID: PMC3616428.
Richardson Lab
For more about Dr. Richardson’s lab, visit her research lab webpage.
Research projects
- DNA damage and translocations caused by bioflavonoids.
- DNA repair pathway choice.
- In vivo models of homologous recombination and non-homologous endjoining to repair double strand breaks.
Representative Publications
- Goodenow D, Emmanuel F, Berman C, Sahyouni M, Richardson C. Bioflavonoids cause DNA double-strand breaks and chromosomal translocations through topoisomerase II-dependent and -independent mechanisms. Mut Res: Gen Tox Env Gen. available online 2020.
- Bariar BB, Vestal CV, Deem B, Goodenow D, Ughetta M, Engledove W, Sahyouni M, Richardson C. Bioflavonoids promote stable translocations between MLL–AF9 breakpoint cluster regions independent of chromosomal context: model system to screen environmental risks. Env. Mol. Mutagenesis, 2019 PMID: 30387535.
- White, R, Sung, P, Vestal, CG, Benedetto, G., Cornelio, N., and Richardson, C. Double-strand break repair by interchromosomal recombination: an in vivo repair mechanism utilized by multiple somatic tissues in mammals. PlosONE, 8(12): 1-16, 2013. e84379. PMID: 24349572 PMCID: PMC3862804
Tomida Lab
For more about Dr. Tomida’s lab, visit his research lab webpage.
Research projects
- Define factors leading to metastasis and chemoresistance in prostate, breast, and ovarian cancers (knockout mouse and cell)
- Characterization of protein complex of DNA repair proteins
- DNA interstrand crosslink repair pathway
Representative Publications
- Tomida J, Takata KI, Bhetawal S, Person MD, Chao HP, Tang DG, Wood RD. FAM35A associates with REV7 and modulates DNA damage responses of normal and BRCA1-defective cells. EMBO J. 2018;37(12). Epub 2018/05/24. doi: 10.15252/embj.201899543. PMID: 29789392. co-corresponding author
- Tomida J, Takata K, Lange SS, Schibler AC, Yousefzadeh MJ, Bhetawal S, Dent SY, Wood RD. REV7 is essential for DNA damage tolerance via two REV3L binding sites in mammalian DNA polymerase ζ. Nucleic Acids Res 43(2):1000-11, 1/2015. e-Pub 1/2015. PMCID: PMC4333420.
- Tomida J, Itaya A, Shigechi T, Unno J, Uchida E, Ikura M, Masuda Y, Matsuda S, Adachi J, Kobayashi M, Meetei AR, Maehara Y, Yamamoto K, Kamiya K, Matsuura A, Matsuda T, Ikura T, Ishiai M, Takata M. A novel interplay between the Fanconi anemia core complex and ATR-ATRIP kinase during DNA cross-link repair. Nucleic Acids Res 41(14):6930-41, 8/2013. e-Pub 5/2013. PMCID: PMC3737553.
Truman Lab
For more about Dr. Truman’s lab, visit his research lab webpage.
Research projects
- Regulation of the DNA damage response by molecular chaperones
- Understanding Hsp70 phosphorylation in cancer
- Using cross-linking mass spectrometry to understand PTM-driven chaperones interactions
Representative Publications
- Xu L, Nitika, Hasin N, Cuskelly DD, Wolfgeher D, Doyle S, Moynagh P, Perrett S, Jones GW, Truman AW*. Rapid deacetylation of yeast Hsp70 mediates the cellular response to heat stress. Sci Rep. 2019;9(1):16260. doi: 10.1038/s41598-019-52545-3. PubMed PMID: 31700027.
- Ricco N, Flor A, Wolfgeher D, Efimova EV, Ramamurthy A, Appelbe OK, Brinkman J, Truman AW, Spiotto MT, Kron SJ. Mevalonate pathway activity as a determinant of radiation sensitivity in head and neck cancer. Mol Oncol. 2019;13(9):1927-43. Epub 2019/06/22. doi: 10.1002/1878-0261.12535. PubMed PMID: 31225926.
- Knighton LE, Delgado LE, Truman AW*. Novel insights into molecular chaperone regulation of ribonucleotide reductase. Curr Genet. 2019;65(2):477-82. Epub 2018/12/07. doi: 10.1007/s00294-018-0916-7. PubMed PMID: 30519713;
- Sluder IT, Nitika, Knighton LE, Truman AW*. The Hsp70 co-chaperone Ydj1/HDJ2 regulates ribonucleotide reductase activity. PLoS Genet. 2018;14(11):e1007462. Epub 2018/11/20. doi: 10.1371/journal.pgen.1007462. PubMed PMID: 30452489.
- Truman AW, Kristjansdottir K, Wolfgeher D, Hasin N, Polier S, Zhang H, Perrett S, Prodromou C, Jones GW, Kron SJ. CDK-dependent Hsp70 Phosphorylation controls G1 cyclin abundance and cell-cycle progression. Cell. 2012;151(6):1308-18. Epub 2012/12/12. doi: 10.1016/j.cell.2012.10.051. PubMed PMID: 23217712.
van Oosten-Hawle Lab
For more about Dr. van Oosten-Hawle’s lab, visit her research lab webpage: https://www.vanoostenhawlelab.com/
Research projects
1. Transcellular chaperone signaling in the regulation of organismal health and disease
2. Gut-to-neuron stress signaling promoting neuroprotective and behavioral responses
3. Organismal proteostasis pathways in the regulation of the pathogen and DNA damage response
Representative Publications
1. Good SC, Dewison KM, Radford SE, van Oosten-Hawle P. Global Proteotoxicity Caused by Human β2 Microglobulin Variants Impairs the Unfolded Protein Response in C. elegans. International Journal of Molecular Sciences. 2021; 22(19):10752.
2. O’Brien, D., Jones, L., Good, S., Miles, J., Aston, R., Smith, C., Vijayabaskar, S., Westhead, D., and van Oosten-Hawle, P. (2018) A PQM-1-mediated response mediates transcellular chaperone signaling and organismal proteostasis. Cell Reports. 2018 Jun 26;23(13):3905-3919
3. Miles, J., van Oosten-Hawle P. Tissue-specific RNAi tools to identify components for systemic stress signaling. Journal of Visual Experiments (JOVE) (2020) May 16;(159).
4. van Oosten-Hawle, P., Porter, R.S., and Morimoto, R. I. (2013). Regulation of organismal proteostasis by transcellular chaperone signaling. Cell 153, 1366-1378.
Yan Lab
For more about Dr. Yan’s lab, visit his research lab webpage
Research projects
- DNA Single-strand break repair and signaling
- Oxidative stress response and redox regulation
- DNA replication stress response in genome stability
- DNA repair and DNA damage response pathways in human diseases (cancer, sepsis, aging, and neurodegenerative diseases)
Representative Publications
- Lin Y, Raj J, Li J, Ha A, Hossain MA, Richardson C, Mukherjee P, Yan S*. 2019. APE1 senses DNA single-strand breaks for repair and signaling. Nucleic Acids Research. (PMID: 31828326; Epub ahead of print) DOI: https://doi.org/10.1093/nar/gkz1175
- Lin Y, Bai L, Cupello S, Hossain MA, Deem B, McLeod M, Raj J, Yan S*. 2018. APE2 promotes DNA damage response pathway from a single-strand break. Nucleic Acids Research. 46 (5): 2479-2494. (PMCID: PMC5861430; PMID: 29361157)
- Wallace BD§, Berman Z§,, Mueller GA, Lin Y, Chang T, Andres SN, Wojtaszek JL, DeRose EF, Appel CD, London RE, Yan S*, Williams RS*. 2017. APE2 Zf-GRF facilitates 3′-5′ resection of DNA damage following oxidative stress. Proceedings of the National Academy of Sciences of the United States of America. 114 (2):304-309. (PMCID: PMC5240719; PMID: 28028224)
- Yan S*, Sorrell M, Berman Z¶. 2014. Functional interplay between ATM/ATR-mediated DNA damage response and DNA repair pathways in oxidative stress. Cellular and Molecular Life Sciences. 71 (20): 3951-3967. (PMCID: PMC4176976; PMID: 24947324)
- Willis J§, Patel Y§, Lentz B, Yan S*. 2013. APE2 is required for ATR-Chk1 checkpoint activation in response to oxidative stress. Proceedings of the National Academy of Sciences of the United States of America. 110 (26): 10592-10597. (PMCID: PMC3696815; PMID: 23754435).
Cancer-omics and computational modeling
With the decreasing costs of the next generation sequencing (NGS) approaches and the advancement of new technologies in the omics arena, a massive amount of cancer omics data have been generated and become available to the research community. For example, The Cancer Genome Atlas (TCGA) has sequenced over 20,000 primary cancer and corresponding normal samples covering 33 cancer types. These big data present a number of great challenges to the downstream analysis for cancer discovery, diagnosis, and treatment: how to efficiently integrate and visualize the data, what are the common and unique signatures or patterns among different cancer types, and how these data inform discovery of better and novel cancer therapeutics. Research areas synergize withthe Bioinformatics Resaerch Center and School of Data Science. Main Areas of Research in Program #2:
- Cancer transcriptomic and metabolomic research
- Data analytics and mathematical modeling
- Data integration and visualization
- Structural bioinformatics and functional studies
Cancer Therapeutics and Health Disparity
Targeted therapies have become the encouraging approaches to prevent cancer progression. However, disparity in tumors sensitivity and patient response and access to such treatments remains a challenge. Through investigations using relevant models including genetically engineered animals, immunocompetent mouse models, 3D cultures, the Cancer Therapeutics and Health Disparity program assesses innovative targeted therapies including immunotherapies, their delivery, diagnostic and monitoring. Moreover, measurement and interventions aim at reducing disparities in cancer treatment and survivorship modeled on efforts that limit diabetes and metabolic disease in the community will be extended to underrepresented cancer patient cohorts. Research areas synergize with the Center for Biomedical Engineering and Science (CBES). Main Areas of Research in Program #3:
- Tumor microenvironment and signaling
- Targeted therapy including immunotherapy
- Nanotechnology and targeted drug delivery
- Cancer disparity, cancer survivorship and community health access
STEM education and community engagement
The benefits of STEM education on the overall learning experience and academic success of undergraduate/graduate students are well documented. The proposed GICI will include the development of course-based research experiences (CUREs) including dissemination of research findings, research internships, and professional development modules. GICI also incorporates multiple STEM education initiatives and professional development programs. GICI faculty are actively engaged in STEM education and outreach with the community through various outreach programs including Discovery Place (public engagement) and HBCUs such as Johnson C. Smith University and NC A&T University (recruitment of graduate students). STEM education and community engagement activities developed by GICI are critical for establishing a large, diverse talent pool to meet the ever-increasing national demand for scientists in this field.